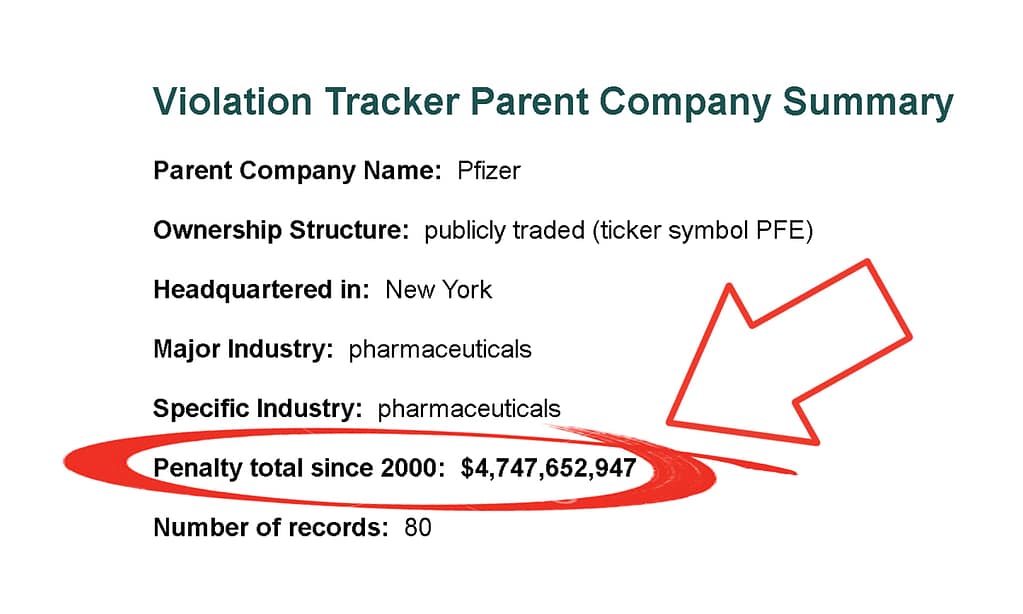
Violation Tracker Parent Company Summary
Parent Company Name: Pfizer Ownership Structure: publicly traded (ticker symbol PFE) Headquartered in: New York Major Industry: pharmaceuticals Specific Industry: pharmaceuticals Penalty total since 2000: $4,660,896,333 Number of records: 71
| Top 5 Offense Groups (Groups Defined) | Penalty Total | Number of Records |
|---|---|---|
| healthcare-related offenses | $3,373,675,000 | 10 |
| government-contracting-related offenses | $1,109,688,435 | 19 |
| safety-related offenses | $104,014,255 | 11 |
| competition-related offenses | $63,466,568 | 6 |
| environment-related offenses | $4,571,885 | 19 |
| Top 5 Primary Offense Types | Penalty Total | Number of Records |
|---|---|---|
| off-label or unapproved promotion of medical products | $3,373,675,000 | 10 |
| False Claims Act and related | $1,109,688,435 | 19 |
| drug or medical equipment safety violation | $103,840,000 | 5 |
| Foreign Corrupt Practices Act | $60,216,568 | 3 |
| environmental violation | $4,571,885 | 19 |
Notes:
Parent-subsidiary linkages are based on relationships current as of the latest revision listed in the Update Log, which may vary from what was the case when a violation occurred. The penalty totals are adjusted to account for the fact that the individual entries below may include both agency records and settlement announcements for the same case; or else a penalty covering multiple locations may be listed in the individual records for each of the facilities. The totals are also adjusted to reflect cases in which federal and state or local agencies cooperated and issued separate announcements of the outcome. Duplicate or overlapping penalty amounts are marked with an asterisk in the list below. Links:
For an overview of this company’s accountability track record, read its Corporate Rap Sheet here.
For more details on misconduct by this company, see its entry in the Project On Government Oversight’s Federal Contractor Misconduct Database.
Subsidy Tracker data on financial assistance to this company by federal, state and local government agencies can be found here.
For an overview of assistance this company is receiving under the CARES Act, see its Covid Stimulus Watch summary page here.
Individual Penalty Records:
Click on the company or penalty amount for more information on each case.
Download results as or XML (maximum 10,000; for access to larger downloads contact Phil Mattera)
| Company | Primary Offense Type | Year | Agency | Penalty Amount |
|---|---|---|---|---|
| Pfizer Inc. | off-label or unapproved promotion of medical products | 2009 | FDA | $2,300,000,000 |
| Pfizer Inc. | False Claims Act and related | 2016 | DOJ_CIVIL | $784,600,000 |
| Wyeth Pharmaceuticals Inc. | off-label or unapproved promotion of medical products | 2013 | FDA | $490,900,000 |
| Warner-Lambert | off-label or unapproved promotion of medical products | 2004 | FDA | $430,000,000 |
| Wyeth Pharmaceuticals, Inc. | False Claims Act and related | 2016 | MULTI-AG | (*) $371,351,180 |
| Pfizer, Inc. | kickbacks and bribery | 2009 | MULTI-AG | (*) $331,485,170 |
| Wyeth Pharmaceuticals, Inc. | off-label or unapproved promotion of medical products | 2013 | MULTI-AG | (*) $257,400,000 |
| Warner-Lambert | off-label or unapproved promotion of medical products | 2004 | MULTI-AG | (*) $190,000,000 |
| King Pharmaceuticals | False Claims Act and related | 2005 | DOJ_CIVIL | $124,000,000 |
| King Pharmaceuticals | False Claims Act and related | 2006 | MULTI-AG | (*) $124,000,000 |
| Pfizer Inc. | off-label or unapproved promotion of medical products | 2008 | MULTI-AG | $60,000,000 |
| Pfizer Inc. | drug or medical equipment safety violation | 2012 | FDA | $55,000,000 |
| Pfizer Corporation | False Claims Act and related | 2002 | DOJ_CIVIL | $49,000,000 |
| Pfizer Inc. | off-label or unapproved promotion of medical products | 2012 | MULTI-AG | $42,900,000 |
| Alpharma Inc. | False Claims Act and related | 2010 | DOJ_CIVIL | $42,500,000 |
| Pfizer Inc. | False Claims Act and related | 2019 | IL-AG | $41,047,101 |
| Pfizer | off-label or unapproved promotion of medical products | 2014 | MULTI-AG | $35,000,000 |
| Wyeth-Ayerst Laboratories Division | drug or medical equipment safety violation | 2000 | FDA | $30,000,000 |
| Pfizer Inc. | Foreign Corrupt Practices Act | 2012 | SEC | $26,339,944 |
| Pfizer, Inc. | False Claims Act and related | 2018 | DOJ_CIVIL | $23,850,000 |
| Pfizer Inc. | False Claims Act and related | 2002 | MULTI-AG | (*) $21,084,700 |
| Wyeth LLC | Foreign Corrupt Practices Act | 2012 | SEC | $18,876,624 |
| Pfizer Inc. | False Claims Act and related | 2013 | TX-AG | $18,170,000 |
| Pfizer H.C.P. Corporation | Foreign Corrupt Practices Act | 2012 | DOJ_CRIMINAL | $15,000,000 |
| Pfizer Inc. | off-label or unapproved promotion of medical products | 2011 | FDA | $14,500,000 |
| Alpharma USPD, Inc. and Purepac Pharmaceutical Co. | False Claims Act and related | 2011 | KY-AG | $10,200,000 |
| Pfizer Inc. | drug or medical equipment safety violation | 2014 | NV-AG | $9,500,000 |
| Alpharma Inc. | False Claims Act and related | 2010 | MULTI-AG | (*) $8,900,000 |
| Pfizer, Inc. and Pharmacia Corporation | False Claims Act and related | 2010 | HI-AG | $8,200,000 |
| Pfizer | drug or medical equipment safety violation | 2003 | MULTI-AG | $6,000,000 |
| Pfizer, Inc. | drug or medical equipment safety violation | 2012 | OR-AG | $3,340,000 |
| Pfizer Inc. and Pharmacia Corp. | False Claims Act and related | 2012 | ID-AG | $2,900,000 |
| Pfizer Inc. | False Claims Act and related | 2011 | MULTI-AG | (*) $2,621,154 |
| Alpharma, Inc. | price-fixing or anti-competitive practices | 2004 | FTC | $2,500,000 |
| King Pharmaceuticals LLC | environmental violation | 2013 | EPA | $2,200,000 |
| KING PHARMACEUTICALS | environmental violation | 2013 | EPA | (*) $2,200,000 |
| Alpharma USPD, Inc., | False Claims Act and related | 2011 | MS-AG | $2,010,667 |
| Purepac Pharmaceutical Co., n/k/a Actavis Elizabeth, LLC | False Claims Act and related | 2011 | MS-AG | $2,010,667 |
| Wyeth | benefit plan administrator violation | 2013 | private lawsuit-federal | $2,000,000 |
| Pfizer | employment discrimination | 2009 | private lawsuit-federal | $1,365,003 |
| Pfizer Inc. | environmental violation | 2008 | EPA | $975,000 |
| Pfizer | consumer protection violation | 2019 | OR-AG | $975,000 |
| PFIZER GLOBAL MANUFACTURING | environmental violation | 2008 | EPA | (*) $975,000 |
| Alpharma, Inc. | price-fixing or anti-competitive practices | 2004 | MULTI-AG | $750,000 |
| Pfizer Pharmaceuticals LLC | environmental violation | 2014 | EPA | $728,000 |
| Pfizer Pharmaceuticals, LLC | environmental violation | 2014 | EPA | (*) $728,000 |
| Pfizer Inc. | consumer protection violation | 2018 | NY-AG | $700,000 |
| Alpharma USPD, Inc. | False Claims Act and related | 2010 | ID-AG | $600,000 |
| Purepac Pharmaceutical Co. | False Claims Act and related | 2010 | ID-AG | $600,000 |
| Hospira Inc. | employment discrimination | 2015 | OFCCP | $400,000 |
| Pfizer Inc. | off-label or unapproved promotion of medical products | 2013 | MA-AG | $375,000 |
| Pfizer Pharmaceuticals, LLC | environmental violation | 2016 | EPA | $190,000 |
| HOSPIRA, INC. | workplace safety or health violation | 2004 | OSHA | $132,300 |
| SEARLE LTD | environmental violation | 2002 | EPA | $95,000 |
| Wyeth Pharmaceuticals Company, Inc. | environmental violation | 2010 | EPA | $77,000 |
| Pfizer Pharmaceuticals LLC | environmental violation | 2005 | EPA | $55,000 |
| Wyeth Rouses Point | environmental violation | 2008 | EPA | $44,500 |
| Pfizer Inc. | environmental violation | 2006 | CT-ENV | $40,224 |
| Wyeth Biopharma | wage and hour violation | 2005 | WHD | $40,187 |
| Wyeth Ayerst Pharmaceuticals | environmental violation | 2004 | EPA | $37,000 |
| Pfizer, Inc. | environmental violation | 2010 | CT-ENV | $31,266 |
| WYETH PHARMACEUTICALS COMPANY – OTC | environmental violation | 2000 | EPA | $25,000 |
| Pfizer Pharmaceuticals | environmental violation | 2010 | EPA | $24,650 |
| PFIZER INC / GROTON SITE | environmental violation | 2005 | EPA | $22,500 |
| Pfizer Pharmaceuticals Ltd. | environmental violation | 2004 | EPA | $20,025 |
| Pfizer Inc. | railroad safety violation | 2002 | FRA | $11,250 |
| PFIZER, INC. | workplace safety or health violation | 2012 | OSHA | $9,600 |
| KING PHARMACEUTICALS | workplace safety or health violation | 2001 | OSHA | $7,200 |
| PFIZER, INC. | workplace safety or health violation | 2009 | OSHA | $7,155 |
| HOSPIRA | workplace safety or health violation | 2006 | OSHA | $6,750 |
| Pfizer Inc. | environmental violation | 2012 | CA-DPR | $6,720 |
Download results as or XML (maximum 10,000; for access to larger downloads contact Phil Mattera)